ARTICLES

Indoor Growers Benefit from Dissolved Oxygen Mastery
MORE Dissolved Oxygen Means MORE Production
Research shows that increasing dissolved oxygen (DO) levels in irrigation water correlate to increases in yield, quality, and overall plant health. A study was conducted by observing the growth of lettuce grown at differing DO and water temperature levels. The study showed at a constant temperate of 18° C an increase in the DO levels from 6.5 to 8.5 mg / L resulted in a 33% increase in yield! [1]
Another study with lettuce grown in deep water culture (DWC) showed similar results. At a high DO level (> 20 mg / L) the total dry mass of the plant was shown to increase up to 2.3 times compared to the control level (8.5 mg / L on average). This study shows that the increases in yields are not solely water weight.[1]
[1] Zan Ouyang, Juncang Tian, Xinfang Yan, Hui Shen, Effects of different concentrations of dissolved oxygen or temperatures on the growth, photosynthesis, yield and quality of lettuce, Agricultural Water Management, Volume 228, 2020 https://www.sciencedirect.com/science/article/pii/S0378377419314933?via%3Dihub [2] A. Suyantohadi, T. Kyoren, M. Hariadi, M.H. Purnomo, T. Morimoto, Effect of high concentrated dissolved oxygen on the plant growth in a deep hydroponic culture under a low temperature, IFAC Proceedings Volumes, Volume 43, Issue 26, 2010, Pages 251-255, https://www.sciencedirect.com/science/article/pii/S1474667015310727?via%3Dihub“Root” Cause Analysis
Plants clearly benefit from increased oxygen availability in the root zone, or Rhizosphere. Within this rhizosphere, the oxygen concentration of greatest physiological interest is at the interface between the root tissue shown in the figure below[3]. This figure shows brown particulate representing the substrate and blue water droplets infiltrating the substrate and root tissue. These water droplets contain various dissolved nutrients and oxygen that are delivered to the rhizosphere during irrigation where they are absorbed by the substrate. The root tissue of the plant then uptakes this solution and its constituents through an osmotic process.
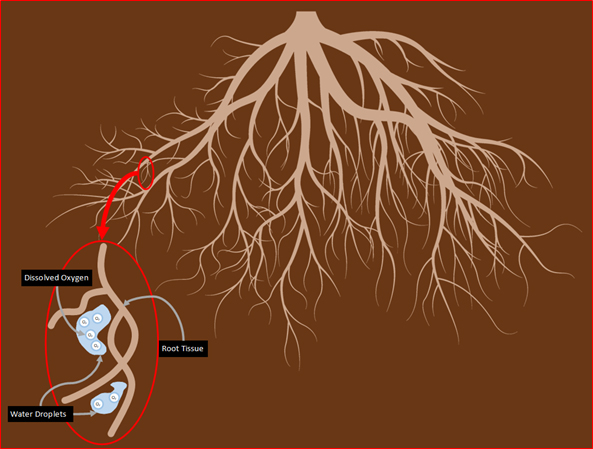
Figure 1: Shows a view of the rhizosphere with a detail near the root tissue’s surface. The blue bubbles represent water droplets that contain dissolved oxygen.
The levels of oxygen dissolved in the solution affect the rate of nutrient and water absorption. Higher DO increases the efficiency of the cellular respiration process, which mainly takes place during light deprivation periods. This process allows cells in the root tissue to process more glucose and therefore uptake more water and nutrients[4]. These improved efficiencies only occur when there are elevated
[3] H. Soffer, D. W. Burger, (1988). Effects of Dissolved Oxygen Concentrations in Aero-hydroponics on the Formation and Growth of Adventitious Roots, 1-4 https://journals.ashs.org/jashs/view/journals/jashs/113/2/article-p218.xml [4] Roblero, M. de J. M., Pineda, J. P., León, M. T. C., & Castellanos, J. S. (2020, May). Oxygen in the root zone and its effect on plants. ResearchGate. Retrieved November 3, 2022, from https://www.researchgate.net/publication/342503181_Oxygen_in_the_root_zone_and_its_effect_on_plantsavailability of oxygen. These benefits to the plant’s health will cease when supplemented oxygen comes out of solution or is consumed by the plant’s root tissue or biofilm. Therefore, growers often monitor their DO levels over time and seek to continually maintain oxygen availability in the rhizosphere throughout the whole plant lifecycle.
Controlling DO levels is vital for growers and has been seen to supply positive results at and above 8 mg / L with healthy roots. With the increase in DO in the rhizosphere, positively charged ions that are dissolved in the nutrient solution will be attracted to the negative electric charges of O2 molecules in the rhizosphere.[5] Typically when DO is less than 2 mg / L, it is low enough for bacteria and pathogens to survive in the rhizosphere. At these low DO levels, issues such as root rot (Pythium) will become prevalent.
Important Factors for Managing Healthy DO Levels
Three primary factors influence oxygen saturation levels in water. These factors are the salinity of the water as measured by its electro-conductivity (EC), the residual oxygen demand of the biofilm in solution, and the temperature for which the water is maintained. All of these factors have an inverse relationship with DO. As any combination of the factors increases, the DO levels will decrease, further decreasing its associated water quality.
1. EC vs. DO
EC measures the salinity of water. Dissolved oxygen has an inverse relationship with salinity as can be seen in Figure 3 below, which shows the effects of increased salinity on DO content. This effect happens as the ionically charged salts in solution attract water molecules that want to bond. The bond formation between the salt ions and the water decreases the solubility of oxygen molecules. This relationship is shown in the figure below.
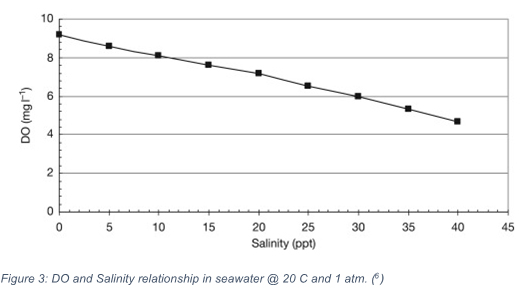
Figure 2: DO and Salinity relationship in seawater @ 20C and 1 atm [6]
[5] J. Park, K. Kurata, (2009). Application of Microbubbles to Hydroponics Solution Promotes Lettuce Growth, 1-4 https://journals.ashs.org/horttech/view/journals/horttech/19/1/article-p212.xml2. Residual Oxygen Demand
Any biofilm present in the water will serve to residually lower the oxygen levels and availability. Biofilm has a metabolism, meaning it is dependent on oxygen to survive and carry out its aerobic process. If oxygen and nutrients exist in abundance, then the biofilm can continue to multiply. When these populations flourish, they decrease the DO residually, thereby lowering the water quality delivered to the plants. Through monitoring and benchmarking DO levels over time, growers can have a fundamental indicator of biofilm contamination and growth. If the typical DO levels begin to drop, it is a good indicator that biofilm may be the culprit.
3. Dissolved Oxygen Potential vs. Water Temperature
Water temperature and the maximum oxygen saturation point have an inverse relationship as shown in the figure below. As water temperature decreases, the solubility of oxygen in water decreases. Therefore, both the rate of dissolution and the concentration of oxygen in the water will increase as the temperature decreases.[6] Typically, between 18°C and 24°C is the optimal range for most plants. This range ensures the temperatures are low enough to support higher DO levels, but not low enough to stress plants from the cold. Every 1 mg / L of DO is equivalent to about a 6°C (11°F) change in temperature.
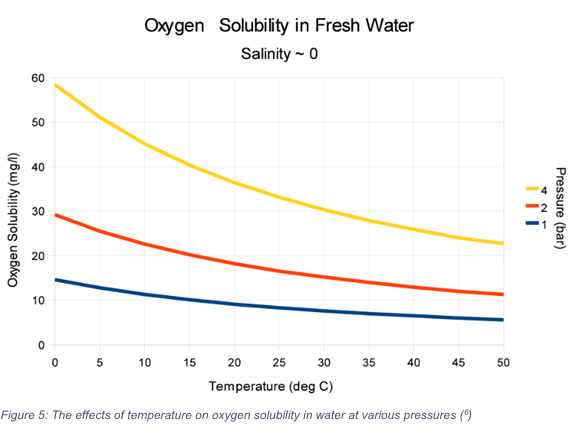
Figure 3: The effects of temperature on oxygen solubility in water at various pressures
[6] Engineering ToolBox, (2005). Oxygen – Solubility in Fresh and Sea Water vs. Temperature. Available at: https://www.engineeringtoolbox.com/oxygen-solubility-water-d_841.html [Accessed 01.11.2022].

